Tartrazine
| |

| |
| Names | |
|---|---|
| IUPAC name
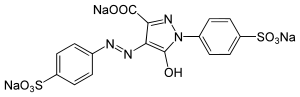
Trisodium 5-hydroxy-1-(4-sulfonatophenyl)-4-[(E)-(4-sulfonatophenyl)diazenyl]-1H-pyrazole-3-carboxylate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.016.091 |
| E number | E102 (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H9N4Na3O9S2 | |
| Molar mass | 534.36 g·mol−1 |
| 20 g/100 mL | |
| Solubility | 18 g/100 mL in glycerol, negligible in ethanol |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tartrazine is a synthetic lemon yellow azo dye primarily used as a food coloring.[1][2][3][4] It is also known as E number E102, C.I. 19140, FD&C Yellow 5, Yellow 5 Lake, Acid Yellow 23, Food Yellow 4, and trisodium 1-(4-sulfonatophenyl)-4-(4-sulfonatophenylazo)-5-pyrazolone-3-carboxylate.[5]
Tartrazine is a commonly used coloring agent all over the world, mainly for yellow, and can also be used with brilliant blue FCF (FD&C Blue 1, E133) or green S (E142) to produce various green shades. It serves as a dye for wool and silks, a colorant in food, drugs and cosmetics and an adsorption-elution indicator for chloride estimations in biochemistry.
History
[edit]Tartrazine was discovered in 1884 by Swiss chemist Johann Heinrich Ziegler, who developed the yellow azo dye in the laboratories of the Bindschedler'sche Fabrik für chemische Industrie in Basel (CIBA). This was patented and produced in Germany by BASF in 1885 (DRP 34294). The process was first presented in 1887 in Chemische Berichte, the journal of the German Chemical Society.[6] Although the structure proposed by Ziegler was not confirmed, he was able to develop an alternative synthesis of tartrazine based on the idea that a hydrazone is the tautomeric form of an azo compound (azo-hydrazo tautomerism). This production process was patented in 1893 (British Patent 5693).[7][8]
Products containing tartrazine
[edit]Foods
[edit]
Many foods contain tartrazine in various proportions, depending on the manufacturer or person preparing the food. When in food, tartrazine is typically labelled as "color", "tartrazine", or "E102", depending on the jurisdiction, and the applicable labeling laws (see Regulation below).
Products containing tartrazine commonly include processed commercial foods that have an artificial yellow or green color, or that consumers expect to be brown or creamy looking. It has been frequently used in the bright yellow coloring of imitation lemon filling in baked goods. The following is a list of foods that may contain tartrazine:[citation needed]
- Desserts and confectionery: ice cream, ice pops and popsicles, confectionery and hard candy (such as gummy bears, marshmallows, etc.), cotton candy, instant puddings and gelatin, cake mixes, pastries, custard powder, marzipan, biscuits, and cookies.
- Beverages: soft drinks, energy and sports drinks, powdered drink mixes, fruit cordials, and flavored/mixed alcoholic beverages.
- Snacks: flavored corn chips (such as nachos, etc.), chewing gum, popcorn (both microwave and cinema-popped), and potato chips.
- Condiments and spreads: jam, jelly (including mint jelly), marmalade, mustard, horseradish, pickles (and other products containing pickles such as tartar sauce and dill pickle dip), and processed sauces.
- Other processed foods: cereal (such as corn flakes, muesli, etc.), instant or "cube" soups, rices (like paella, risotto, etc.), noodles, pureed fruit and pickled peppers, bright-green-colored seaweed salad.
Personal care and cosmetics products
[edit]A number of personal care and cosmetics products may contain tartrazine, usually labelled as CI 19140 or FD&C Yellow 5, including:
- Liquid and bar soaps, green hand sanitizer, moisturizers and lotions, mouth washes, perfumes, toothpastes, and shampoos, conditioners and other hair products.
- Cosmetics, such as eyeshadow, blush, face powder and foundation, lipstick, etc. – even those that are primarily pink or purple. (Usually make-up manufacturers use one label for all shades in a product line, placing the phrase "may contain" ahead of all colors that are used in that line, not necessarily that specific shade.)
- Nail polish, nail polish remover, temporary tattoos, and tanning lotions.
Medications
[edit]Various types of medications include tartrazine to give a yellow, orange or green hue to a liquid, capsule, pill, lotion, or gel, primarily for easy identification.[9] Types of pharmaceutical products that may contain tartrazine include vitamins, antacids, cold medications (including cough drops and throat lozenges), lotions and prescription drugs.
Most, if not all, medication data sheets are required to contain a list of all ingredients, including tartrazine. Some include tartrazine in the allergens alert section.
The Canadian Compendium of Pharmaceuticals and Specialties (CPS), a prescribing reference book for health professionals, mentions tartrazine as a potential allergy for each drug that contains tartrazine.
Other products
[edit]Other products, such as household cleaning products, paper plates, pet foods, crayons, inks for writing instruments, stamp dyes, face paints, envelope glues, and deodorants, may also contain tartrazine.
Chemistry
[edit]Tartrazine is water-soluble[10] and has a maximum absorbance in an aqueous solution at 425 nm.[11] It is one of the oldest known members of the pyrazolone family of dyes.[12]
Potential health effects on humans
[edit]Food intolerance, sensitivity, and allergies.
[edit]This section may be confusing or unclear to readers. (November 2024) |
Tartrazine appears to cause the most allergic and intolerance reactions of all the azo dyes, particularly among asthmatics and those with an aspirin intolerance.[medical citation needed] Symptoms from tartrazine sensitivity can occur by either ingestion or cutaneous exposure to a substance containing tartrazine. Symptoms appear after periods of time ranging from minutes up to 14 hours.[13]
The prevalence of tartrazine intolerance is estimated at 360,000 U.S. Citizens affected, less than 0.12% of the general population.[14] According to the FDA, tartrazine causes hives in fewer than 1 in 10,000 people, or 0.01%.[15]
It is not clear how many individuals are sensitive or intolerant to tartrazine, but the University of Guelph estimates that it is 1 to 10 out of every ten thousand people (0.01% to 0.1% of the population).[16] There is much controversy about whether tartrazine has ill effects on individuals who are not clearly intolerant.[citation needed]
Total avoidance is the most common way to deal with tartrazine sensitivity,[17] but progress has been made in reducing people's tartrazine sensitivity in a study of people who are simultaneously sensitive to both aspirin and tartrazine.[18]
Asthma
[edit]A systematic review of the medical literature concluded that, among patients with asthma, research has shown that exposure to tartrazine does not worsen symptoms and avoidance of tartrazine does not improve symptoms; however, "due to the paucity of evidence, it is not possible to provide firm conclusions as to the effects of tartrazine on asthma control".[19]
Food intolerance and ADHD-like behavior
[edit]Tartrazine is one of various food colors said to cause food intolerance and ADHD-like behavior in children.[20] It is possible that certain food colorings may act as a trigger in those who are genetically predisposed, but the evidence for this effect is weak.[21][22]
Reproductive
[edit]Rumors began circulating about tartrazine in the 1990s regarding a link to its consumption (specifically its use in Mountain Dew) and alleged adverse effects on male erectile function, testicle and penis size, and sperm count.[23][24] The rumors likely because as the result of an in vitro study showing an effect on estrogen receptors; however this effect was disproven in later in vivo studies. Tartrazine may have a reproductive effect at extremely high dosages, however it has no reproductive effect at the levels found in food.[25]
Regulation
[edit]North America
[edit]Canada
[edit]Tartrazine is listed as a permitted food coloring in Canada.[26] The majority of pre-packaged foods are required to list all ingredients, including all food additives such as color; however section B.01.010 (3)(b) of the Regulations provide food manufacturers with the choice of declaring added color(s) by either their common name or simply as "colour".[27]
In February 2010, Health Canada consulted the public and manufacturers on their plans to change the labelling requirements. Health Canada felt that it might be prudent to require the identification of specific colors on food labels, to allow consumers to make better informed choices.[28] The results of the consultation supported increased transparency.[29] Some respondents proposed banning the use of synthetic food colors, however Health Canada found that existing scientific literature does not demonstrate that synthetic food coloring is unsafe in the general population; they are instead considering more transparent labelling to allow those with sensitivities to food color to make informed choices. The relevant proposed regulatory changes will be developed and published for consultation in Part I of the Canada Gazette,[30] the official newsletter of the Government of Canada.
United States
[edit]The United States requires the presence of tartrazine to be declared on food and drug products (21 CFR 74.1705 (revised April 2013), 21 CFR 201.20) and also color batches to be preapproved by the United States Food and Drug Administration (FDA).[15] As part of these regulations, the FDA requires that the Precautions section of prescription drug labels include the warning statement, "This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity."[31]
The FDA regularly seizes products if found to be containing undeclared tartrazine, declared but not FDA-tested, or labeled something other than FD&C yellow 5 or Yellow 5. Such products seized often include noodles.[32]
Europe
[edit]European Union
[edit]The European Food Safety Authority allows for tartrazine to be used in processed cheese, canned or bottled fruit or vegetables, processed fish or fishery products, and wines and wine-based drinks.[33][34]
The European regulatory community, with a stronger[compared to?] emphasis on the precautionary principle, required labelling and temporarily reduced the acceptable daily intake (ADI) for the food colorings; the UK FSA called for voluntary withdrawal of the colorings by food manufacturers.[21] However, in 2009 the EFSA re-evaluated the data at hand and determined that "the available scientific evidence does not substantiate a link between the color additives and behavioral effects."[21][35]
Tartrazine is among six artificial colors for which the European Union requires products that contain them to be marked with the statement May have an adverse effect on activity and attention in children.[36]
Austria and Germany
[edit]Yellow tartrazine (E102) was banned in Austria[37] and Germany, before European Parliament and Council Directive 94/36/EC lifted the ban.
Norway
[edit]Yellow tartrazine (E102) is banned in Norway (not an E.U. member.)[38][39]
United Kingdom
[edit]In response to concerns about the safety of certain food additives, the UK FSA commissioned a study by researchers at Southampton University of the effect of a mixture of six food dyes (Tartrazine, Allura Red, Ponceau 4R, Quinoline Yellow WS, Sunset Yellow and Carmoisine (dubbed the "Southampton 6")) and sodium benzoate (a preservative) on children in the general population, who consumed them in beverages; the study published in 2007.[21] The study found "a possible link between the consumption of these artificial colours and a sodium benzoate preservative and increased hyperactivity" in the children;[21][40] the advisory committee to the FSA that evaluated the study also determined that because of study limitations, the results could not be extrapolated to the general population, and further testing was recommended.[21]
In 2008 Scotland asked for Scottish food producers voluntarily stop using these food dyes.[40] A 2010 study found that one third of food producers were still using at least one of the Southhampton Six.[41]
Other uses
[edit]3D printing
[edit]Tartrazine has been used as a biocompatible photoblocker for generating transparent hydrogels with complex inner structures.[42]
Tissue imaging
[edit]Ou et al.[43] initially reported that tartrazine can make living tissues transparent by tuning the refractive index of the surrounding medium to match that of the cells;[44] however, they later withdrew their paper after it was discovered the transparent tissue was, in fact, dead, and tartrazine can only make damaged cells transparent.[45]
See also
[edit]- Sunset yellow FCF, also known as Yellow 6
References
[edit]- ^ Food Standards Australia New Zealand. "Food Additives- Numerical List". Archived from the original on June 25, 2009. Retrieved 2 December 2009.
- ^ Current EU approved additives and their E Numbers, Food Standards Agency website, retrieved 15 Dec 2011
- ^ "Food Dyes". Center for Science in the Public Interest. Archived from the original on 6 July 2016. Retrieved 8 March 2013.
- ^ "What is Food Coloring Made Of?". WiseGeek. Retrieved 8 March 2013.
- ^ "Acid Yellow 23". ChemBlink, an online database of chemicals from around the world.
- ^ Johann Heinrich Ziegler, M. Locher (1887), "Ueber die Tartrazine, eine neue Klasse von Farbstoffen" [On the tartrazines, a new class of dyes], Berichte der Deutschen Chemischen Gesellschaft, vol. 20, no. 1, pp. 834–840, doi:10.1002/cber.188702001188
- ^ H.E. Fierz (1936), Hans Schinz (ed.), "Johann Heinrich Ziegler (1857-1936)" [Quarterly Journal of the Natural Science Society in Zurich] (PDF), Vierteljahresschrift der Naturforschenden Gesellschaft in Zürich, vol. 83. Jahrgang, Heft 3 und 4, Zürich, pp. 313–314
- ^ R. Anschütz (1897), "Ueber die Constitution des Tartrazins" [On the constitution of tartrazine], Justus Liebig's Annalen der Chemie, vol. 294, no. 2, pp. 219–243, doi:10.1002/jlac.18972940207
- ^ "Tartrazine". drugs.com.
- ^ Sigma-Aldrich. "SIGMA CHEMICAL COMPANY -- T0388 TARTRAZINE -- 6550-00F051158". siri.org. Archived from the original on 2016-03-03.
- ^ Jain, Rajeev; Bhargava, Meenakshi; Sharma, Nidhi (2003). "Electrochemical Studies on a Pharmaceutical Azo Dye: Tartrazine". Industrial & Engineering Chemistry Research. 42 (2): 243–247. doi:10.1021/ie020228q.
- ^ Hunger K, Herbst W (2012). "Pigments, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_371. ISBN 978-3527306732.
- ^ Alvarez Cuesta E, Alcover Sánchez R, Sainz Martín T, Anaya Turrientes M, García Rodríguez D (Jan–Feb 1981). "[Pharmaceutical preparations which contain tartrazine]". Allergol Immunopathol (Madr). 9 (1): 45–54. PMID 7258046.
- ^ Elhkim MO, Héraud F, Bemrah N, et al. (April 2007). "New considerations regarding the risk assessment on Tartrazine: An update toxicological assessment, intolerance reactions and maximum theoretical daily intake in France". Regulatory Toxicology and Pharmacology. 47 (3): 308–316. doi:10.1016/j.yrtph.2006.11.004. PMID 17218045.
- ^ a b "Does FD&C Yellow No. 5 cause any allergic reactions?". United States Food and Drug Administration. Archived from the original on 2007-10-09. Retrieved 2007-10-20.
- ^ "Artificial Colours | Food Safety Network". web.archive.org. 2014-04-07. Retrieved 2024-11-16.
- ^ Dipalma JR (November 1990). "Tartrazine sensitivity". American Family Physician. 42 (5): 1347–50. PMID 2239641.
- ^ Michel O, Naeije N, Bracamonte M, Duchateau J, Sergysels R (May 1984). "Decreased sensitivity to tartrazine after aspirin desensitization in an asthmatic patient intolerant to both aspirin and tartrazine". Annals of Allergy. 52 (5): 368–70. PMID 6721262.
- ^ Ardern KD, Ram FS (2014-01-24). "Tartrazine exclusion for allergic asthma". Cochrane Database Syst Rev. 2017 (4): CD000460. doi:10.1002/14651858.CD000460. PMC 6483719. PMID 11687081.
- ^ Spencer, Peter; Barret, Emily; Taioli, Emanuela (April 2021). "Potential Neurobehavioral Effects of Synthetic Food Dyes in Children" (PDF). California Office of Environmental Health Hazard Assessment.
- ^ a b c d e f FDA. Background Document for the Food Advisory Committee: Certified Color Additives in Food and Possible Association with Attention Deficit Hyperactivity Disorder in Children: March 30–31, 2011
- ^ Millichap JG, Yee MM (February 2012). "The diet factor in attention-deficit/hyperactivity disorder". Pediatrics. 129 (2): 330–337. doi:10.1542/peds.2011-2199. PMID 22232312. S2CID 14925322.
- ^ Bahloul, Maria (August 25, 2016). "Investigating the Middle School Rumor that Mountain Dew Lowers Your Sperm Count". Vice.
- ^ "Mountain Dew Shrinks Testicles". snopes.com. 14 October 1999. Retrieved 2012-11-10.
- ^ Amchova, Petra; Siska, Filip; Ruda-Kucerova, Jana (2024-09). "Safety of tartrazine in the food industry and potential protective factors". Heliyon. 10 (18): e38111. doi:10.1016/j.heliyon.2024.e38111. PMC 11458953. PMID 39381230.
Importantly, all the studies reporting potential reproductive risks used high doses, which cannot be ingested from food sources. Tartrazine does not seem to exert any reproductive toxicity under the current ADI.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Table III of section B.16.100, Food and Drug Regulations
- ^ Branch, Legislative Services (2022-01-05). "Consolidated federal laws of canada, Food and Drug Regulations". laws-lois.justice.gc.ca. Retrieved 2022-02-03.
- ^ "Health Canada Proposal to Improve Food Colour Labelling Requirements". Health Canada. 2010-01-28. Retrieved 15 June 2012.
- ^ Health Canada reviews comments received on the proposed changes to current food colour labelling regulations for prepackaged foods
- ^ Canada Gazette
- ^ CFR – Code of Federal Regulations Title 21
- ^ ORA (May 2, 2013). "Import Alert 45-02". fda.gov. Retrieved May 5, 2013.
- ^ "further details can be found on the EFSA food additives database page on tartrazine". Archived from the original on 2014-04-07. Retrieved 2014-04-06.
- ^ "FOODS". Archived from the original on 2014-04-07. Retrieved 2014-04-06.
- ^ EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) (November 2009). "Scientific Opinion on the re-evaluation Tartrazine (E 102)". EFSA Journal. 7 (11): 1331–1382. doi:10.2903/j.efsa.2009.1331.
The Panel concludes that the present dataset does not give reason to revise the ADI of 7.5 mg/kg bw/day.
- ^ "Food additives". Food Standards Agency. Retrieved 2018-10-31.
- ^ "Taste the rainbow forever: yellow mac & cheese is dead, but the nostalgia lives on". the Guardian. 21 April 2015. Retrieved 4 September 2022.
- ^ Firman, Tehrene (21 August 2022). "American Food Products Banned In Other Countries". Eat This Not That.
- ^ "Taste the rainbow forever: yellow mac & cheese is dead, but the nostalgia lives on". the Guardian. 21 April 2015. Retrieved 4 September 2022.
- ^ a b Sarah Chapman of Chapman Technologies on behalf of Food Standards Agency in Scotland. (March 2011), Guidelines on approaches to the replacement of Tartrazine, Allura Red, Ponceau 4R, Quinoline Yellow, Sunset Yellow and Carmoisine in food and beverages (PDF), archived from the original (PDF) on May 2014
{{citation}}: Check date values in:|archive-date=(help) Cite error: The named reference "FSAguideline" was defined multiple times with different content (see the help page). - ^ "Evaluation of the progress made by Scottish SMEs with the voluntary withdrawal of the 'Southampton Six' Colours from food products". Food Standards Scotland. Retrieved 2024-11-16.
{{cite web}}: zero width space character in|title=at position 1 (help) - ^ Aaron, Benjamin (January 2019). "Light-based 3D Printing of Hydrogels with High-resolution Channels". Biomedical Physics & Engineering Express. 5 (2): 025035. doi:10.1088/2057-1976/aad667.
- ^ Ou, Zihao; Duh, Yi-Shiou; Rommelfanger, Nicholas J.; Keck, Carl H. C.; Jiang, Shan; Brinson, Kenneth; Zhao, Su; Schmidt, Elizabeth L.; Wu, Xiang; Yang, Fan; Cai, Betty; Cui, Han; Qi, Wei; Wu, Shifu; Tantry, Adarsh (2024-09-06). "Achieving optical transparency in live animals with absorbing molecules". Science. 385 (6713): eadm6869. Bibcode:2024Sci...385m6869O. doi:10.1126/science.adm6869. ISSN 0036-8075. PMID 39236186.
- ^ Rowlands, Christopher J.; Gorecki, Jon (2024-09-06). "Turning tissues temporarily transparent". Science. 385 (6713): 1046–1047. Bibcode:2024Sci...385.1046R. doi:10.1126/science.adr7935. ISSN 0036-8075. PMID 39236198.
- ^ Inagaki, Shigenori; Imai, Takeshi (2024-10-02), WITHDRAWN: Tartrazine cannot make live tissues transparent, doi:10.1101/2024.09.29.615648, retrieved 2024-11-16

